Abstract
Unlike pediatric cases, adult patients with myeloid neoplasia (MN) are less often suspected to harbor germline (GL) driver or predisposition alterations. However, recent discoveries of various genes affected by GL alterations linked to long disease anticipation and incomplete penetrance indicate a growing role of familial predisposition in adult MN including, the impact of heterozygous alleles within recessive traits, biallelic (GL/somatic) or compound heterozygous (GL/GL and GL/somatic) configurations of various genes.
Recently, genomic profiling of 3 early-onset AMLs identified MBD4 GL loss as an initiator of 5-methylcytosine (5mC)-dependent hypermutation with a 33-fold higher mutational burden (>95% being C>T in the context of a CG)1. In cases from our institution, a biallelic MBD4 c.1291C>T, p.Arg431* & c.1688T>A p.Leu563* (in trans) tracked with AML in brother and sister2. MBD4 is a known DNA glycohydrolase involved in DNA mismatch repair (MMR) essential for reducing genotoxic stress in normal cells. Loss of function mutation in MBD4 may result in compromised MMR leading to genomic instability. MBD4 binds to 5mC and 5-hydroxymethylcytosine (5hmC), oxidation products of TET2 dioxygenases, and initiates a multi-step MMR around methylated CpG DNA. Therefore, we hypothesized that loss of MBD4 may result in reduced MMR leading to increased genomic instability and acquisition of additional lesions which may facilitate clonal evolution.
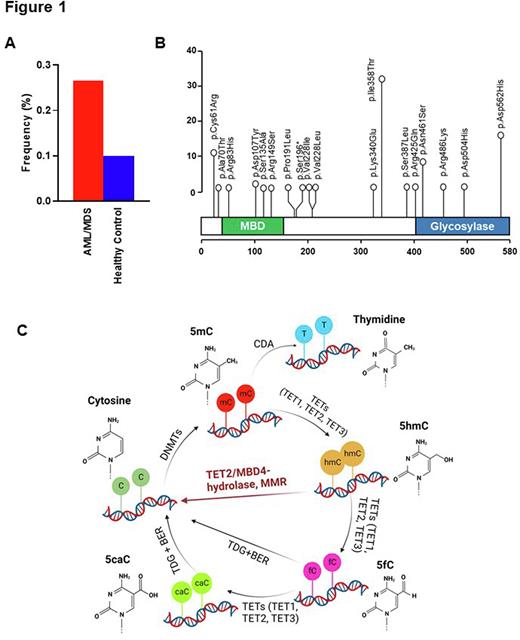
We first analyzed data from 1,692 MN patients (732 MDS and 960 AML) and found 134 (8%) patients with 22 MBD4 variants. Overall, 20 were non-synonymous missense (in 132 pts), while nonsense and frameshift deletion were also observed in 1 case each. After variant filtering and annotation, we retained 17 recurrent missense in 80 patients and 1 nonsense variant in 1 patient at an odds ratio of >1.5 vs. healthy control, for a total of 81 patients harboring MBD4 VUS (Fig.1A). Among them, 4 variants (p.Arg83His, p.Asp107Tyr, p.Ser135Ala, p.Arg149Ser) mapped within the methyl-CpG-binding domain, whereas 5 variants (p.Arg425Gln, p.Asn461Ser, p.Arg486Lys, p.Asp504His, p.Asp562His) were located in the glycosylase domain (Fig.1B). Of note is that all cases were heterozygous. Comparing MBD4MTvs.MBD4WT cases, there was no significant difference in age (median was 69 vs. 71 yrs), diagnosis, clinical parameters (WBC, ANC, Hb, Plt counts, peripheral and bone marrow blast percentage) and past history of malignancy. When we compared the somatic genotype of MBD4 VUS carriers vs. carriers of MBD4WT, somatic TET2 hits were found with a significantly lower frequency (15 vs. 25%, P=.046), in particular in AML (7 vs. 22%, P=.009) and in cases with TET2 truncations (9 vs. 19%, P=.018).
To further investigate the biochemical basis of MBD4 dysfunction, and its connection to TET2 DNA dioxygenases, we knocked out MBD4 in TET2 mutant and in isogenic control cell lines. To test if the DNA hydrolase activity impacts on genomic accumulation of 5hmC in TET2-dependent manner, we assessed the impact of MBD4 loss on DNA methylation using 5mC and 5hmC ratio as read out in MBD4KO and WT cells in TET2MT and isogenic controls. Our results show that the loss of MBD4 resulted in increased 5hmC compared with the isogenic WT cells when accompanied with TET2WT but this effect was absent in TET2KO cells (mean fold change, 1.9±0.4 vs. 0.9±0.1; P=.03), suggesting a cross talk between MBD4 and TET2. Consequently, in agreement with our hypothesis, TET2WT cells with loss of MBD4 were significantly more sensitive to olaparib-mediated PARP1 inhibition. We also performed the RNA-seq analysis of MBD4WT and KO cells in the presence and absence of functional TET2.
These results demonstrate that MBD4 variant carrier status may be a predisposition factor for the development of MN. As loss of MBD4 mimicked the loss of TET-dioxygenase function, MBD4 could function downstream of TET2 converting 5mC to 5hmC and to cytosine as a DNA damage repair associated protein (Fig. 1C). Our findings pave the way for consideration of MBD4 as a potential actionable target to regulate DNA methylation in association with TET2 during leukemogenesis.
Disclosures
Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Maciejewski:Alexion: Consultancy; Apellis Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.